Endometrial cancer clinical trials
Women saved by endometrial cancer clinical trials
"The trial gave her the opportunity to have a therapy that she wouldn't otherwise have had access to"

When Patricia Hawkins was diagnosed with endometrial cancer in 2016 and the cancer later returned despite radiation therapy and surgery, “I kind of lost it there,” she remembered. Hawkins takes care of her three young granddaughters. “I was doing a lot of crying. When you have cancer, it’s deadly, there’s no turning back.”
Her physician, Linda Diska, MD, of the University of Virginia Health system talked to her about enrolling in a clinical trial.
“The trial gave her the opportunity to have a therapy that she wouldn’t otherwise have had access to,” Duska explains. “Patricia was fortunate enough to have a really good response to the treatment and stayed on for almost three years with a really good quality of life and some really good time with her family.”
“So far, so good,” says Patricia. “I feel pretty good, move around, do what I need to do.”
A 5-minute video from the American Association for Cancer Research.
"I am so thankful. It has been the only treatment that has consistently kept my cancer from spreading."

In the fall of 2016, Pam Matthews began experiencing a lingering, unusual pain when she crossed her legs or slept on her stomach. An ultrasound revealed a surprising diagnosis: uterine (endometrial) cancer.
A hysterectomy, chemotherapy and radiation kept the cancer from spreading for only about six months before it reached her lungs, requiring more surgery. She joined two clinical trials, but the cancer kept coming back.
Then in 2020 Pam learned about a clinical trial testing a new oral drug that targeted specifically her tumor’s gene mutation and might stop what was driving her tumor’s growth.
“Treatment had left me so weak physically and mentally frustrated. My husband and I jumped for joy when we learned we had another option. It gave us hope, and to date it has been the only treatment that has consistently kept my cancer from spreading. I am so thankful,” says Pam.
Now age 72, Pam has stable tumors, and no additional ones have developed. She spends much of her free time with her extended family, including three grandchildren, two step-grandchildren and two great-grandchildren.
"When I take my tablets twice a day, I may be taking them by myself, but I am not alone."

Because all of the effort, the energy, the intention, the desire, the plan, the background, the training of brilliant people, whether they are the researchers or my medical team, everyone had a role in my amazingly good outcome.”
Source: Society of Gynecologic Oncology.
"I participated in a clinical trial and I mattered not only in my own health, but in others as well."
“You get this opportunity to participate in a clinical trial where you’re saying that I made a difference and so therefore you’re not only empowered, but you’re saying that it mattered.
I mattered. I participated in a clinical trial and I mattered not only in my own health, but in others as well. Because I will always have been a participant. And that’s something that someone can’t take from you.”
A 1-minute video from the Society of Gynecologic Oncology.
What are clinical trials and why join one?
Cancer clinical trials allow researchers to study innovative and potentially life-saving new treatments. The goal is to find treatments that are better than what’s currently available.
Placebos are rarely used in cancer treatment clinical trials. If participants don’t receive the study medication, they are always given the best available treatment for their particular cancer. This helps the researchers determine whether the study medication is better than the standard treatment.
A 2-minute video from Stand Up to Cancer
Reason one. You have a chance to receive treatment based on the latest research that is not yet available to others. Clinical trials are based on the best knowledge available for your kind of cancer.
Reason two. You’ll get high-quality care and be carefully monitored throughout the study.
Reason three. You add to the world’s knowledge about cancer and help to improve cancer care for future generations.
A 2-minute video from the National Cancer Institute
All of today's standard cancer treatments are the result of clinical trials conducted years ago
If you have been diagnosed with a gynecologic cancer, your doctor may have mentioned clinical trials. Clinical trials test new medical treatments and procedures. In eligible patients, participation is strictly voluntary.
In clinical trials, patients are monitored closely for their response to the treatment. Experienced researchers use the study data to help these patients and other women with gynecologic cancers expand their range of treatment options.
All of today’s standard cancer treatments are the result of clinical trials conducted years ago, And each of the new and promising cancer treatments that come along are also a result of clinical trial research, which means that patients on existing clinical trials have the opportunity to receive the therapies of tomorrow.
A 4-minute video from the Foundation for Women’s Cancer.
Gynecologic cancer clinical trials an option at any stage of treatment from initial diagnosis to recurrence to survivorship
When you’ve been diagnosed with a gynecologic cancer, you may find yourself in unfamiliar territory every step of the way. There are decisions to be made.
Knowing your options can be a breath of fresh air in the midst of cancer treatment. Whether you’ve been newly diagnosed or have undergone several different therapies.
Joining a clinical trial is another choice that you can make.
An in-depth explanation of clinical trials from the Society of Gynecologic Oncology and the Foundation for Women’s Cancer. This 19-minute video won a Gold EXCEL Award in Digital Media: Video (Education) from Association Media & Publishing.

“There are some well founded reasons why there is mistrust in research because of well documented historical abuses,” says Lannis Hall, M.D.
“I have told patients for years, if they want to ensure the absolute best care, then they want to request participation on a clinical study. It is because the safeguards in clinical trials are multilayered.”
“Clinical trials test new drugs or new regimens or different ways of treating cancer against the current standard to see if the new interventions would be better than what we currently have,” says Foluso Bisi Ademuyiwa, M.D.
A 5-minute promotional video for the Siteman Cancer Center in St. Louis, one of 71 National Cancer Institute-Designated Cancer Centers in the United States.

“We know that patients who are enrolled on studies today typically have a better outcome than those who are not enrolled in studies. They’re going to be getting nothing less than the standard of care,” says Jeff Michalski, M.D.
“And often the oversight and quality assurance that goes along with that leads to a better outcome.”

From 1988 to 2019, clinical trials in gynecologic malignancies rarely reported the race and ethnicity of patients, according to findings from an analysis led researchers at Duke University. The study evaluated 1,882 clinical trials and found that less than 10 percent reported the race of patients, making it difficult to track minority representation in trials.
Where data were available, White women made up more than 80% of the enrolled population in trials for ovarian, uterine, vulvar, and vaginal cancer, and 65.2% of the population in trials for cervical cancer. In endometrial cancer trials, only seven percent of participants were Black and six percent were Hispanic.
“The first step in addressing cancer care disparities involves transparency in reporting enrollment of racial and ethnic minorities in clinical trials among women with gynecologic cancers,” the researchers wrote. “Reporting of race and ethnicity should be required for all clinical trials. This data could be used to promote a standard table on race and ethnicity that could be included in all future trials.”
Source: “Race, Ethnicity ‘Notably Underreported’ in Publicly Accessible Databases of Gynecologic Cancer Trials” (2022)
How else will we know that a new treatment works as well for all races, ethnicities, ages and genders?

Clinical trials provide individuals with cancer the opportunity to participate in groundbreaking research that may bring about new treatments that improve quality of life, extend survival, and even prove lifesaving. Research and results that come from clinical trials also shed light on the side effects that participants experience during the study.
If people from all groups are not represented in clinical trials, we will not know if a new cancer treatment works as well for all ages, genders, races, and ethnic groups. We also will not know if some groups are more likely than other groups to develop side effects.
If all groups are not represented in clinical trials, they will not have the chance to benefit equally from the newest treatments. This can widen the differences we see in cancer outcomes in the U.S.
Source: “The Importance of Diversity in Clinical Trials,” an 8-page brochure from The Cancer Support Community (2021)
Critical information is gained only by participating in clinical trials
“I always try to explain to my patients the importance for minorities to participate in the clinical trials so that we’re represented in data analysis,” says Patricia Robinson, M.D., a cancer physician-researcher and Associate Dean in the Stritch School of Medicine at Loyola University, Maywood, Illinois.
“We don’t all metabolize drugs the same way and I think it’s important for people to recognize, within their patient population based upon genetics, what the potential benefit may be as well as the potential toxicities. If you are a slow metabolizer or quick metabolizer, that information is only gained by participating in clinical trials and having the data analyzed.”
A 2-minute video from the Cancer Support Community.


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.

A free, over-the-phone service that helps Black or African American (AA) cancer patients learn more about clinical trials.
The Peer Clinical Trials Support Program matches interested patients with a peer — a Black or African American cancer patient or survivor with experience participating in a cancer clinical trial. Patients have the opportunity to discuss their fears, questions, and concerns with a knowledgeable and empathetic guide, and hear from someone of a similar background who has “been there, done that.”
Source: Cancer Support Community
National Cancer Institute

If you are thinking about taking part in a clinical trial, be sure to ask your doctor, “Is there a clinical trial that I can join?” If your doctor offers you a trial, here are some questions you may want to ask, such as:
How long will I be in the trial?
What kinds of tests and treatments are involved?
How will the doctor know if the treatment is working?
How will I be told about the trial’s results?
How long do I have to make up my mind about joining this trial?
Who can I speak with about questions I have during and after the trial?
Who will be in charge of my care?
Is there someone I can talk to who has been in the trial?
More at: “Questions to Ask Your Doctor about Treatment Clinical Trials” (National Cancer Institute)
American Cancer Society

- What phase is this clinical trial in?
- Why is this study being done?
- How long do I have to make this decision?
- What’s likely to happen if I decide to take part or not take part in the clinical trial?
- Will the researchers work with my cancer doctor? Who will be in charge of my care?
- Who will I get in touch with if I have problems, questions, or concerns?
- What are my other options (standard treatments, other clinical trials)? What are the pros and cons of each?
- How much do you know about this treatment? About clinical trials in general?
- What were the results in past studies of this treatment? How likely are they to apply to me?
- Is there anything else I can read about this clinical trial?
- What kinds of treatments and tests would I need to have? How often are they done?
- Would I need to plan on extra time or travel?
And more questions at “Deciding Whether to Be Part of a Clinical Trial” by the American Cancer Society (2022)
Steps to Find a Clinical Trial
Learn how to find the right clinical trial for you with the National Cancer Institute’s six-step guide:
Step 1: Gather Details about Your Cancer
Step 2: Find Clinical Trials
Step 3: Take a Closer Look at the Trials that Interest You
Step 4: Contact the Team Running the Trial
Step 5: Ask Questions
Step 6: Make an Appointment
A 2-minute video from the National Cancer Institute
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted in all 50 states and in 221 countries.
The website provides current information about clinical research studies for patients, their families and caregivers, health care professionals, and the public.
Each study record includes a summary of the study protocol, including the purpose, recruitment status, and eligibility criteria. Study locations and specific contact information are listed to assist with enrollment.
Information on ClinicalTrials.gov is provided and updated by the organizations and people that sponsor and carry out the studies. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Clinicaltrials.gov is a free service of the National Institutes of Health (NIH) and is maintained by the National Library of Medicine (NLM).
For more information about using the database, visit clinicaltrials.gov

“If you decide to look for trials on your own, learn how to find the right clinical trial for you with the National Cancer Institute’s six-step guide:
- Step 1: Gather details about your cancer
- Step 2: Find clinical trials
- Step 3: Take a closer look at the trials that interest you
- Step 4: Contact the team running the trial
- Step 5: Ask questions
- Step 6: Make an appointment
The NCI’s Cancer Information Service can also provide a tailored clinical trials search that you can discuss with your doctor. To reach them call 1-800-4-CANCER (1-800-422-6237) and select option 2. This is a free service. Keep in mind that the search results do not replace advice from your doctor.
Source: National Cancer Institute (2022)
Help from non-profit 501(c)3 charitable organizations


Our Cancer Immunotherapy Clinical Trial Finder will aid you in finding your answer to cancer. Understand the basics of cancer clinical trials, why clinical trials are so critical to our work, what things to consider about enrolling, and how to assist patients in finding clinical trials for which they may be eligible.
Source: Cancer Research Institute, a 501(c)3 charitable organization.
Standup to Cancer
Source: Stand up to Cancer, a division of the Entertainment Industry Foundation (EIF), a 501(c)(3) charitable organization.

If you are a cancer patient who needs help identifying clinical trial options or needs financial assistance to attend a clinical trial, we’d like to hear from you. Our Patient Navigator can help you find the right clinical trial. We can also help you with the costs associated with participating in a clinical trial, including transportation and lodging.
For more information, email Lazarex or to speak with a Lazarex representative, call 925.820.4517.
Source: Lazarex Cancer Foundation, a 501(c)3 charitable organization.


Finding the right trial can be overwhelming. Search Clinical Trials is a free service designed to help people find clinical trials that are relevant to their medical and healthcare needs.
The CISCRP team will work with you to understand your options and help you find local clinical trials in your community, or as far as you would be comfortable traveling.
Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.
Help from private companies
For over a decade, EmergingMed’s clinical navigators have facilitated clinical trial searches for nearly 500,000 patients. Request a Contact today to learn how we are able to help.
Source: EmergingMed

“As a digital patient engagement company, Antidote’s focus is on connecting patients to clinical trials — that’s why we have a moral imperative to support diversity, equity, and inclusion (DEI) initiatives that ensure clinical trial populations reflect those of the real world.”
Source: Antidote
“Our site has a comprehensive listing of all trials in the United States and is categorized to help you find it quick. ClinicalTrialsGPS has information on trials from all the leading pharmaceutical companies, academic institutions and trials ranging from Cancer to Diabetes and Vaccines to Osteoarthritis.”
“Our goal is to provide you with the most comprehensive, up to date information about ongoing clinical studies as well as a quick and easy way to participate. “
Source: Clinical Trials GPS
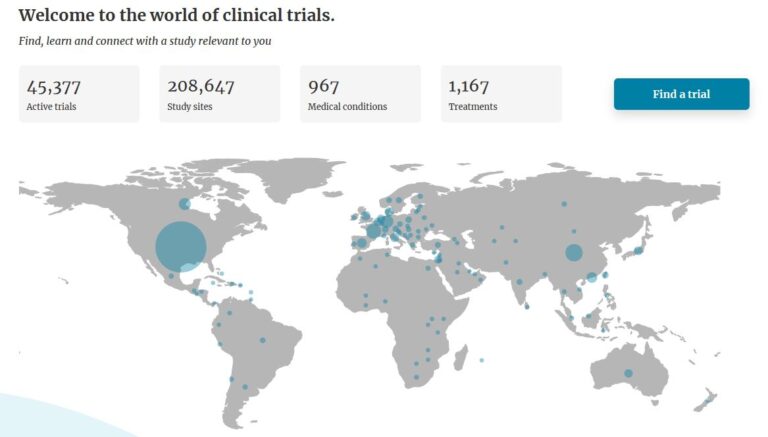
Search and browse to find a trial relevant to you. Read the trial details on eligibility, location and participation. You can also register to be notified when a trial you’re searching for begins. Find a trial near you.
Source: Center Watch