Colorectal cancer clinical trials
Saved by colorectal cancer clinical trials
"You saved my life on that phone call."

Stephanie was sitting with her parents, “just completely, I mean discouraged doesn’t begin to explain it, but I was just ready to give up,” when Luis Diaz, M.D., called from the Kimmel Cancer Center at Johns Hopkins University and told her to get to Baltimore as soon as she could. “We’ve been been having a lot of success with patients like you.”
Stephanie had stage 4 colorectal cancer that had returned for the third time and after two surgeries was now inoperable. When her physicians in Philadelphia conceded there was nothing more they could do for her, Stephanie’s younger sister scoured the Internet looking for help and found Dr. Diaz’ immunotherapy clinical trial at Johns Hopkins.
The call from Dr. Diaz offering her a place in his clinical trial “was a lifeline because I didn’t have anything to hold on to at that time at all,” Stephanie recalls. “You saved my life on that phone call.”
In the clinical trial, she had a major response to the immunotherapy and says her tumor shrank by 60% to 65% within three months. Two years later, her scan showed no evidence of cancer.
“I haven’t felt this well in five years, which is obviously beyond incredible,” Stephanie marvels. “But it does take a little bit of time for me to fully comprehend how well I’m feeling because it was really difficult for so long.”
A wonderful conversation between Stephanie and Luis Diaz, M.D., in a 13-minute video from the Kimmel Cancer Center at Johns Hopkins University.
“It’s been a journey and with each step, I’m just so grateful that I’m still living.”

Vanessa Johnson Brandon (above on the right, with her cancer physician Dung Le, M.D.) awoke early on a morning in June 2014, with stomach pain, vomiting and diarrhea. A hospital emergency room took a CT scan, which revealed a large mass in her colon. The news terrified her because her own mother had died of breast cancer at the age of 56.
Vanessa had colon cancer which already spread to her liver. Recovery was slow after surgery, so her doctors started her on a potent brew of chemotherapy. Every two weeks, Vanessa underwent three straight days of grueling IV chemo administered at her home. Never really feeling better, after 11 months her doctor told her there was nothing more he could do for her.
Vanessa’s husband jumped into action and found a clinical trial underway near their home, testing a promising new immunotherapy treatment. Johns Hopkins researchers were looking for patients with a certain genetic profile they thought would benefit the most from the treatment. Only about one person in eight would qualify, but Vanessa’s gene mutations “matched up perfectly” and she was told to come in right away to start her bloodwork. “I cried so hard I saw stars,” she recalled.
After a year and a half of treatment with the experimental drug, which was Keytruda, Vanessa’s tumors shrank by 66 percent. She still tires easily and has trouble walking due to the nerve damage caused by her earlier rounds of chemotherapy. Still, she thinks of every day as a blessing. “It’s been a journey and with each step, I’m just so grateful that I’m still living.”
Source: “Could Immunotherapy Lead the Way to Fighting Cancer?” by Robin Marantz Henig on the Smithsonian magazine website.
“Because of the clinical trial, I have a life with my children. “Now I can think a little bit toward the future.

Corina Ramirez, diagnosed with colon cancer in 2015, had surgery to remove tumors from her colon and aorta, followed by 13 rounds of chemotherapy. At the time, her daughter was just a few months old and her son was 4.
Treatment for advanced colon cancer took its toll. Physically and emotionally drained from chemotherapy, she was unable to care for her family the way she wanted.
When her cancer was no longer responding to chemotherapy, a friend advised her to contact the University of Kansas Cancer Center, the region’s only National Cancer Institute-designed cancer center, about a clinical trial.
Corina was eligible to enroll in an innovative clinical trial that was showing promising results in patients with Corina’s particular type of genetic disease, which was micro-satellite instability causing a deficiency in a cancer cell’s ability to repair its DNA.
Once in the study, she says she knew almost immediately the treatment was working. Eight weeks later, a scan revealed her tumor had shrunk by nearly 50%. Her progress continues at a slower but steady pace, and she says the immunotherapy has dramatically improved her quality of life.
“Because of the clinical trial, I have a life with my children,” she shares. “Now I can think a little bit toward the future, but I don’t take anything for granted. Every day is a blessing.”
What are clinical trials and why join one?
Cancer clinical trials allow researchers to study innovative and potentially life-saving new treatments. The goal is to find treatments that are better than what’s currently available.
Placebos are rarely used in cancer treatment clinical trials. If participants don’t receive the study medication, they are always given the best available treatment for their particular cancer. This helps the researchers determine whether the study medication is better than the standard treatment.
A 2-minute video from Stand Up to Cancer
Reason one. You have a chance to receive treatment based on the latest research that is not yet available to others. Clinical trials are based on the best knowledge available for your kind of cancer.
Reason two. You’ll get high-quality care and be carefully monitored throughout the study.
Reason three. You add to the world’s knowledge about cancer and help to improve cancer care for future generations.
A 2-minute video from the National Cancer Institute
Clinical trials are used for all types and stages of colorectal cancer.
Doctors and scientists are always looking for better ways to care for people with colorectal cancer. To make scientific advances, doctors create research studies involving volunteers, called clinical trials. Every drug that is now approved by the U.S. Food and Drug Administration (FDA) was tested in clinical trials.
Clinical trials are used for all types and stages of colorectal cancer. Many focus on new treatments to learn if a new treatment is safe, effective, and possibly better than the existing treatments. These types of studies evaluate new drugs, different combinations of treatments, new approaches to radiation therapy or surgery, and new methods of treatment.
People who participate in clinical trials can be some of the first to get a treatment before it is available to the public. However, there are some risks with a clinical trial, including possible side effects and the chance that the new treatment may not work. People are encouraged to talk with their health care team about the pros and cons of joining a specific study.
Some clinical trials study new ways to relieve symptoms and side effects during treatment. Others study ways to manage the late effects that may happen a long time after treatment. Talk with your doctor about clinical trials for symptoms and side effects.
Source: Cancer.net from the American Society of Clinical Oncology

“There are some well founded reasons why there is mistrust in research because of well documented historical abuses,” says Lannis Hall, M.D.
“I have told patients for years, if they want to ensure the absolute best care, then they want to request participation on a clinical study. It is because the safeguards in clinical trials are multilayered.”

“Clinical trials test new drugs or new regimens or different ways of treating cancer against the current standard to see if the new interventions would be better than what we currently have,” says Foluso Bisi Ademuyiwa, M.D.
A 5-minute promotional video for the Siteman Cancer Center in St. Louis, one of 71 National Cancer Institute-Designated Cancer Centers in the United States.

“We know that patients who are enrolled on studies today typically have a better outcome than those who are not enrolled in studies. They’re going to be getting nothing less than the standard of care,” says Jeff Michalski, M.D.
“And often the oversight and quality assurance that goes along with that leads to a better outcome.”
Lack of diversity in colorectal cancer clinical trials
Whites have dominated colorectal cancer clinical trials
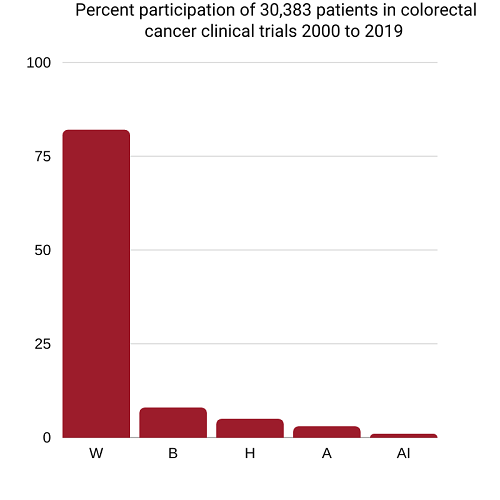
W=White 81.8%, B=Black 8.1%, H=Hispanics 5.1%, Asian/Pacific Islanders 3.4%, American Indian/Alaska Native 0.4%
Black and Hispanic patients under-represented in cutting-edge colorectal cancer clinical trials
Precision oncology clinical trials test potential treatments targeted to specific genetic mutations that may be driving tumor growth. In this study of 14 colorectal cancer trials that were conducted between 2004 and 2017, researchers calculated for racial/ethnic groups the ratio of their participation in the trials to their prevalence of colorectal cancer in the population.
 Black representation in the trials was 40 percent lower and Hispanic representation 39 per cent lower than their share of colorectal cancer in the U.S. population. On the other hand, White representation was 22 percent higher and Asian representation 29 percent higher than it should have been if it had matched their share of colorectal cancer.
Black representation in the trials was 40 percent lower and Hispanic representation 39 per cent lower than their share of colorectal cancer in the U.S. population. On the other hand, White representation was 22 percent higher and Asian representation 29 percent higher than it should have been if it had matched their share of colorectal cancer.
“Current personalized treatments that are broadly generalized to all individuals based on the presence or absence of a biomarker without fully studying the implications of such treatment in racial and ethnic minority populations may not be appropriate,” Sophia C Kamran, M.D., (left) of the Massachusetts General Hospital and her colleagues wrote.
Source: “Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies” (2021).
Why diversity is so important in colorectal cancer clinical trials
How else will we know that a new treatment works as well for all races, ethnicities, ages and genders?

Clinical trials provide individuals with cancer the opportunity to participate in groundbreaking research that may bring about new treatments that improve quality of life, extend survival, and even prove lifesaving. Research and results that come from clinical trials also shed light on the side effects that participants experience during the study.
If people from all groups are not represented in clinical trials, we will not know if a new cancer treatment works as well for all ages, genders, races, and ethnic groups. We also will not know if some groups are more likely than other groups to develop side effects.
If all groups are not represented in clinical trials, they will not have the chance to benefit equally from the newest treatments. This can widen the differences we see in cancer outcomes in the U.S.
Source: “The Importance of Diversity in Clinical Trials,” an 8-page brochure from The Cancer Support Community (2021)
Critical information is gained only by participating in clinical trials
“I always try to explain to my patients the importance for minorities to participate in the clinical trials so that we’re represented in data analysis,” says Patricia Robinson, M.D., a cancer physician-researcher and Associate Dean in the Stritch School of Medicine at Loyola University, Maywood, Illinois.
“We don’t all metabolize drugs the same way and I think it’s important for people to recognize, within their patient population based upon genetics, what the potential benefit may be as well as the potential toxicities. If you are a slow metabolizer or quick metabolizer, that information is only gained by participating in clinical trials and having the data analyzed.”
A 2-minute video from the Cancer Support Community.
"We have to ensure that any medications, any treatments work on all groups."
“Unfortunately African Americans are disproportionately affected by colorectal cancer. We get colorectal cancer more often and are more likely to die from colorectal cancer. There are ways in which we can remedy this,” says Cedrek McFadden, M.D., colorectal surgeon and Clinical Associate Professor at the University of South Carolina School of Medicine Greenville.
“One is help us find new ways to treat colorectal cancer and one of the big ways in which we do that is through clinical trials. Now, I know that many people distrust and really don’t believe in clinical trials. We’re in a different time. We have to understand that clinical trials are highly regulated to your success.
We have to find more effective treatments. And the way in which we do this is through clinical trials because we have to ensure that any medications, any treatments work on all groups. So that is why we have to have the inclusion of African Americans, not only performing the clinical trials but also participating in the clinical trials.
A 1-minute video from the Colorectal Cancer Alliance.
Information about clinical trial participation for people of color


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.

A free, over-the-phone service that helps Black or African American (AA) cancer patients learn more about clinical trials.
The Peer Clinical Trials Support Program matches interested patients with a peer — a Black or African American cancer patient or survivor with experience participating in a cancer clinical trial. Patients have the opportunity to discuss their fears, questions, and concerns with a knowledgeable and empathetic guide, and hear from someone of a similar background who has “been there, done that.”
Source: Cancer Support Community
Questions to ask before joining a clinical trial
National Cancer Institute
 If you are thinking about taking part in a clinical trial, be sure to ask your doctor, “Is there a clinical trial that I can join?” If your doctor offers you a trial, here are some questions you may want to ask, such as:
If you are thinking about taking part in a clinical trial, be sure to ask your doctor, “Is there a clinical trial that I can join?” If your doctor offers you a trial, here are some questions you may want to ask, such as:
More at: “Questions to Ask Your Doctor about Treatment Clinical Trials” (National Cancer Institute)
American Cancer Society

- What phase is this clinical trial in?
- Why is this study being done?
- How long do I have to make this decision?
- What’s likely to happen if I decide to take part or not take part in the clinical trial?
- Will the researchers work with my cancer doctor? Who will be in charge of my care?
- Who will I get in touch with if I have problems, questions, or concerns?
- What are my other options (standard treatments, other clinical trials)? What are the pros and cons of each?
- How much do you know about this treatment? About clinical trials in general?
- What were the results in past studies of this treatment? How likely are they to apply to me?
- Is there anything else I can read about this clinical trial?
- What kinds of treatments and tests would I need to have? How often are they done?
- Would I need to plan on extra time or travel?
And more questions at “Deciding Whether to Be Part of a Clinical Trial” by the American Cancer Society (2022)
Help finding cancer clinical trials from government agencies
clinicaltrials.gov from the National Library of Medicine
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted in all 50 states and in 221 countries.
The website provides current information about clinical research studies for patients, their families and caregivers, health care professionals, and the public.
Each study record includes a summary of the study protocol, including the purpose, recruitment status, and eligibility criteria. Study locations and specific contact information are listed to assist with enrollment.
Information on ClinicalTrials.gov is provided and updated by the organizations and people that sponsor and carry out the studies. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Clinicaltrials.gov is a free service of the National Institutes of Health (NIH) and is maintained by the National Library of Medicine (NLM).
For more information about using the database, visit clinicaltrials.gov
National Cancer Institute

“If you decide to look for trials on your own, learn how to find the right clinical trial for you with the National Cancer Institute’s six-step guide:
- Step 1: Gather details about your cancer
- Step 2: Find clinical trials
- Step 3: Take a closer look at the trials that interest you
- Step 4: Contact the team running the trial
- Step 5: Ask questions
- Step 6: Make an appointment
The NCI’s Cancer Information Service can also provide a tailored clinical trials search that you can discuss with your doctor. To reach them call 1-800-4-CANCER (1-800-422-6237) and select option 2. This is a free service. Keep in mind that the search results do not replace advice from your doctor.
Source: National Cancer Institute (2022)
Help from non-profit 501(c)3 charitable organizations
Cancer Research Institute's Clinical Trial Finder


Our Cancer Immunotherapy Clinical Trial Finder will aid you in finding your answer to cancer. Understand the basics of cancer clinical trials, why clinical trials are so critical to our work, what things to consider about enrolling, and how to assist patients in finding clinical trials for which they may be eligible.
Source: Cancer Research Institute, a 501(c)3 charitable organization.
Standup to Cancer
Source: Stand up to Cancer, a division of the Entertainment Industry Foundation (EIF), a 501(c)(3) charitable organization.
Lazarex Cancer Foundation

If you are a cancer patient who needs help identifying clinical trial options or needs financial assistance to attend a clinical trial, we’d like to hear from you. Our Patient Navigator can help you find the right clinical trial. We can also help you with the costs associated with participating in a clinical trial, including transportation and lodging.
For more information, email Lazarex or to speak with a Lazarex representative, call 925.820.4517.
Source: Lazarex Cancer Foundation, a 501(c)3 charitable organization.


Finding the right trial can be overwhelming. Search Clinical Trials is a free service designed to help people find clinical trials that are relevant to their medical and healthcare needs.
The CISCRP team will work with you to understand your options and help you find local clinical trials in your community, or as far as you would be comfortable traveling.
Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.
Help from private companies
EmergingMed
For over a decade, EmergingMed’s clinical navigators have facilitated clinical trial searches for nearly 500,000 patients. Request a Contact today to learn how we are able to help.
Source: EmergingMed
Antidote

“As a digital patient engagement company, Antidote’s focus is on connecting patients to clinical trials — that’s why we have a moral imperative to support diversity, equity, and inclusion (DEI) initiatives that ensure clinical trial populations reflect those of the real world.”
Source: Antidote
Clinical Trials GPS
“Our site has a comprehensive listing of all trials in the United States and is categorized to help you find it quick. ClinicalTrialsGPS has information on trials from all the leading pharmaceutical companies, academic institutions and trials ranging from Cancer to Diabetes and Vaccines to Osteoarthritis.”
“Our goal is to provide you with the most comprehensive, up to date information about ongoing clinical studies as well as a quick and easy way to participate. “
Source: Clinical Trials GPS
CenterWatch
Search and browse to find a trial relevant to you. Read the trial details on eligibility, location and participation. You can also register to be notified when a trial you’re searching for begins. Find a trial near you.
Source: Center Watch