Cervical cancer clinical trials

“I was diagnosed on Halloween of 2011 with stage 1B2 cervical cancer and after I completed treatment I had clean scans,” says Sue Scott. But when her cancer returned three months later, her physician told her there was no chemotherapy, radiation, or surgery for her at that point. Her outside chance would be an immunotherapy clinical trial if one was available.
“I said what’s immunotherapy? I’ve never heard of it.” She found out that it’s a type of cancer treatment where the patient’s own immune system is used to fight the cancer. A clinical trial was found at the National Institutes of Health and Sue was able to join it.
“Hope was a really important thing. This trial offered a tiny glimmer of hope for me and that was important. There’s so much that takes away from your hope when you’re sick and just having a little glimmer of hope and being able to hold onto that is really important.”
Sue ended up getting lucky. Of the 16 participants in the trial, she was one of the two who responded to the new treatment. “I had dozens of measurable tumors, liver, bladder, kidney pelvis, lymph nodes, all cancerous and in two months, all clean on a scan.”
She hadn’t expected to be cured. “Really it was more about how can I help science? How can I help the next person who ends up here?”
A 5-minute video from the National Cancer Institute.
Cancer clinical trials allow researchers to study innovative and potentially life-saving new treatments. The goal is to find treatments that are better than what’s currently available.
Placebos are rarely used in cancer treatment clinical trials. If participants don’t receive the study medication, they are always given the best available treatment for their particular cancer. This helps the researchers determine whether the study medication is better than the standard treatment.
A 2-minute video from Stand Up to Cancer

Only 3 new cervical cancer treatments have been approved over the last 20 years. Surprising, isn’t it?
The treatments that we have happen to work well for many patients. But there are patients who have a high risk of recurrence, regardless of their stage. Recurrence depends more on lymph node involvement than stage of cancer. So women with stage 1 may be harder to treat than women in stage 4 because of their lymph nodes. And we don’t have medications that work well in women once they’ve had a recurrence. So there is work to do!
All this being said, in order to develop new treatments, we need to have clinical trials. The truth is, clinical research is the only way we ever can get any medications for any of our health conditions.
We need clinical trials to drive progress. We need trials to determine the safety and effectiveness of every type of treatment. And in order to determine that safety and effectiveness, we need volunteers.
Source: “Treatment and Clinical Trials” on the Cervivor website.
Reason one. You have a chance to receive treatment based on the latest research that is not yet available to others. Clinical trials are based on the best knowledge available for your kind of cancer.
Reason two. You’ll get high-quality care and be carefully monitored throughout the study.
Reason three. You add to the world’s knowledge about cancer and help to improve cancer care for future generations.
A 2-minute video from the National Cancer Institute

“There are some well founded reasons why there is mistrust in research because of well documented historical abuses,” says Lannis Hall, M.D.
“I have told patients for years, if they want to ensure the absolute best care, then they want to request participation on a clinical study. It is because the safeguards in clinical trials are multilayered.”
“Clinical trials test new drugs or new regimens or different ways of treating cancer against the current standard to see if the new interventions would be better than what we currently have,” says Foluso Bisi Ademuyiwa, M.D.
A 5-minute promotional video for the Siteman Cancer Center in St. Louis, one of 71 National Cancer Institute-Designated Cancer Centers in the United States.

“We know that patients who are enrolled on studies today typically have a better outcome than those who are not enrolled in studies. They’re going to be getting nothing less than the standard of care,” says Jeff Michalski, M.D.
“And often the oversight and quality assurance that goes along with that leads to a better outcome.”

Hispanic women were 70%, Black women 49%, and Asian women 37% under-represented in 61 ovarian, endometrial and cervical cancer precision oncology clinical trials compared with the expected number based on their prevalence of these cancers in the United States. White women were 26% over-represented.
Precision oncology clinical trials test potential treatments targeted to specific genetic mutations that may be driving tumor growth.
Source: “Enrollment of Individuals From Racial and Ethnic Minority Groups in Gynecologic Cancer Precision Oncology Trials” (2022)

Among 1,882 clinical trials enrolling patients with ovarian, uterine or endometrial, cervical, vaginal, and vulvar cancers from 1988 to 2019, less than 10 percent reported the racial composition of the participants, according to a study by Mary Katherine Montest de Oca of Duke University and her colleagues.
Of these, the racial distribution of enrollees was:
- 66.9% White
- 8.6% Asian
- 8.5% Black/African American
- 0.4% Indian/Alaskan Native
- 0.1% Native Hawaiian/Pacific Islander
- 1.0% more than one race, and
- 14.5% unknown.
Except for cervical cancer trials which enrolled 65% White patients, enrollees in trials for other gynecologic cancers were over 80% White. Reporting of race in gynecologic clinical trials has increased substantially from 2000 to 2018, from 1.7% to 13.9% and and for ethnicity 1.7% to 11.1%.
Source: “Diversity and transparency in gynecologic oncology clinical trials” by Mary Katherine Montes de Oca et al. (2023)
How else will we know that a new treatment works as well for all races, ethnicities, ages and genders?

Clinical trials provide individuals with cancer the opportunity to participate in groundbreaking research that may bring about new treatments that improve quality of life, extend survival, and even prove lifesaving. Research and results that come from clinical trials also shed light on the side effects that participants experience during the study.
If people from all groups are not represented in clinical trials, we will not know if a new cancer treatment works as well for all ages, genders, races, and ethnic groups. We also will not know if some groups are more likely than other groups to develop side effects.
If all groups are not represented in clinical trials, they will not have the chance to benefit equally from the newest treatments. This can widen the differences we see in cancer outcomes in the U.S.
Source: “The Importance of Diversity in Clinical Trials,” an 8-page brochure from The Cancer Support Community (2021)
Critical information is gained only by participating in clinical trials
“I always try to explain to my patients the importance for minorities to participate in the clinical trials so that we’re represented in data analysis,” says Patricia Robinson, M.D., a cancer physician-researcher and Associate Dean in the Stritch School of Medicine at Loyola University, Maywood, Illinois.
“We don’t all metabolize drugs the same way and I think it’s important for people to recognize, within their patient population based upon genetics, what the potential benefit may be as well as the potential toxicities. If you are a slow metabolizer or quick metabolizer, that information is only gained by participating in clinical trials and having the data analyzed.”
A 2-minute video from the Cancer Support Community.


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.


Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.

A free, over-the-phone service that helps Black or African American (AA) cancer patients learn more about clinical trials.
The Peer Clinical Trials Support Program matches interested patients with a peer — a Black or African American cancer patient or survivor with experience participating in a cancer clinical trial. Patients have the opportunity to discuss their fears, questions, and concerns with a knowledgeable and empathetic guide, and hear from someone of a similar background who has “been there, done that.”
Source: Cancer Support Community

- What phase is this clinical trial in?
- Why is this study being done?
- How long do I have to make this decision?
- What’s likely to happen if I decide to take part or not take part in the clinical trial?
- Will the researchers work with my cancer doctor? Who will be in charge of my care?
- Who will I get in touch with if I have problems, questions, or concerns?
- What are my other options (standard treatments, other clinical trials)? What are the pros and cons of each?
- How much do you know about this treatment? About clinical trials in general?
- What were the results in past studies of this treatment? How likely are they to apply to me?
- Is there anything else I can read about this clinical trial?
- What kinds of treatments and tests would I need to have? How often are they done?
- Would I need to plan on extra time or travel?
And more questions at “Deciding Whether to Be Part of a Clinical Trial” by the American Cancer Society (2022)
ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted in all 50 states and in 221 countries.
The website provides current information about clinical research studies for patients, their families and caregivers, health care professionals, and the public.
Each study record includes a summary of the study protocol, including the purpose, recruitment status, and eligibility criteria. Study locations and specific contact information are listed to assist with enrollment.
Information on ClinicalTrials.gov is provided and updated by the organizations and people that sponsor and carry out the studies. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Clinicaltrials.gov is a free service of the National Institutes of Health (NIH) and is maintained by the National Library of Medicine (NLM).
For more information about using the database, visit clinicaltrials.gov

“If you decide to look for trials on your own, learn how to find the right clinical trial for you with the National Cancer Institute’s six-step guide:
- Step 1: Gather details about your cancer
- Step 2: Find clinical trials
- Step 3: Take a closer look at the trials that interest you
- Step 4: Contact the team running the trial
- Step 5: Ask questions
- Step 6: Make an appointment
The NCI’s Cancer Information Service can also provide a tailored clinical trials search that you can discuss with your doctor. To reach them call 1-800-4-CANCER (1-800-422-6237) and select option 2. This is a free service. Keep in mind that the search results do not replace advice from your doctor.
Source: National Cancer Institute (2022)
Help from non-profit 501(c)3 charitable organizations


Our Cancer Immunotherapy Clinical Trial Finder will aid you in finding your answer to cancer. Understand the basics of cancer clinical trials, why clinical trials are so critical to our work, what things to consider about enrolling, and how to assist patients in finding clinical trials for which they may be eligible.
Source: Cancer Research Institute, a 501(c)3 charitable organization.
Standup to Cancer
Source: Stand up to Cancer, a division of the Entertainment Industry Foundation (EIF), a 501(c)(3) charitable organization.

If you are a cancer patient who needs help identifying clinical trial options or needs financial assistance to attend a clinical trial, we’d like to hear from you. Our Patient Navigator can help you find the right clinical trial. We can also help you with the costs associated with participating in a clinical trial, including transportation and lodging.
For more information, email Lazarex or to speak with a Lazarex representative, call 925.820.4517.
Source: Lazarex Cancer Foundation, a 501(c)3 charitable organization.


Finding the right trial can be overwhelming. Search Clinical Trials is a free service designed to help people find clinical trials that are relevant to their medical and healthcare needs.
The CISCRP team will work with you to understand your options and help you find local clinical trials in your community, or as far as you would be comfortable traveling.
Source: Center for Information and Study on Clinical Research Participation, Inc.(CISCRP), a non-profit 501(c)(3) organization advocating for clinical research education.
Help from private companies
For over a decade, EmergingMed’s clinical navigators have facilitated clinical trial searches for nearly 500,000 patients. Request a Contact today to learn how we are able to help.
Source: EmergingMed

“As a digital patient engagement company, Antidote’s focus is on connecting patients to clinical trials — that’s why we have a moral imperative to support diversity, equity, and inclusion (DEI) initiatives that ensure clinical trial populations reflect those of the real world.”
Source: Antidote
“Our site has a comprehensive listing of all trials in the United States and is categorized to help you find it quick. ClinicalTrialsGPS has information on trials from all the leading pharmaceutical companies, academic institutions and trials ranging from Cancer to Diabetes and Vaccines to Osteoarthritis.”
“Our goal is to provide you with the most comprehensive, up to date information about ongoing clinical studies as well as a quick and easy way to participate. “
Source: Clinical Trials GPS
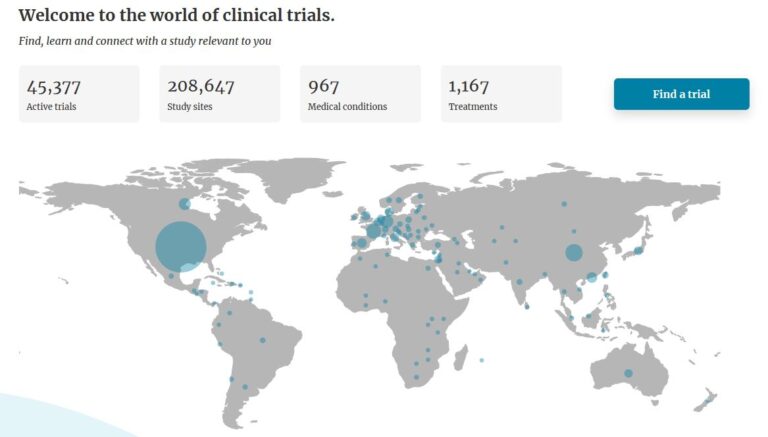
Search and browse to find a trial relevant to you. Read the trial details on eligibility, location and participation. You can also register to be notified when a trial you’re searching for begins. Find a trial near you.
Source: Center Watch